
On December 1, 2020, the United Kingdom (U.K.) became the first western country to give emergency approval for a COVID-19 vaccine. The Medicines and Healthcare Products Regulatory Agency (MHRA) officials announced they would begin distributing 800,000 doses of Pfizer’s coronavirus vaccine to 50 British hospitals as early as next week. The initial vaccines will be administered to some of the nation's most vulnerable citizens — nursing home residents, health workers, and the elderly.
“Today’s emergency use authorization in the U.K. marks a historic moment in the fight against COVID-19. This authorization is a goal we have been working toward since we first declared that science will win, and we applaud the MHRA for their ability to conduct a careful assessment and take timely action to help protect the people of the U.K.,” Albert Bourla, chairman and CEO of Pfizer, said in a press release.
The BNT162b2 vaccine, a collaboration between Pfizer and Germany's BioNTech, is among the two leading inoculations that have shown an over 90 percent vaccine efficacy — the reduction in COVID-19 incidences in injected patients — in clinical trials. The second, labeled mRNA-1273, is being developed by American biotechnology company Moderna Therapeutics.
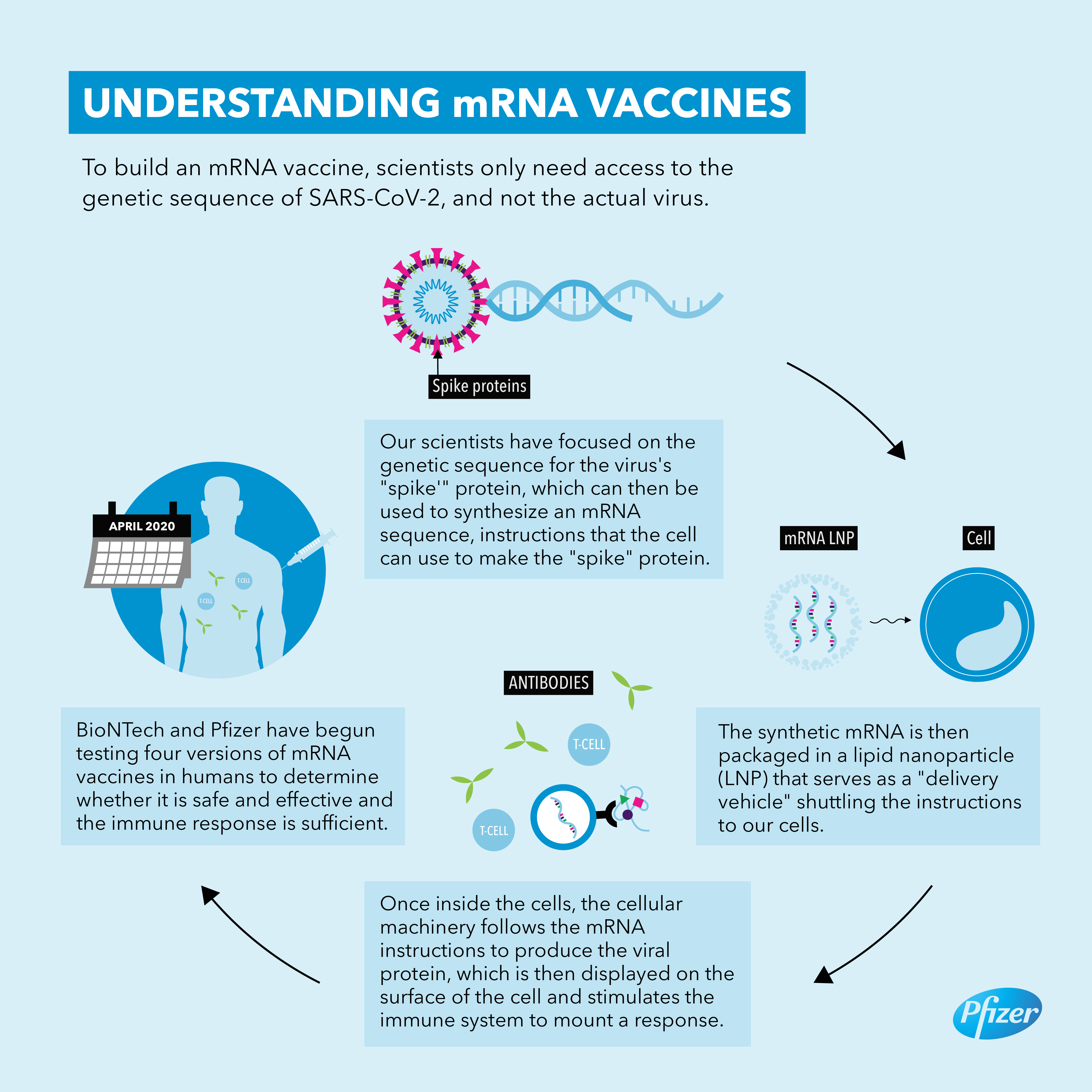
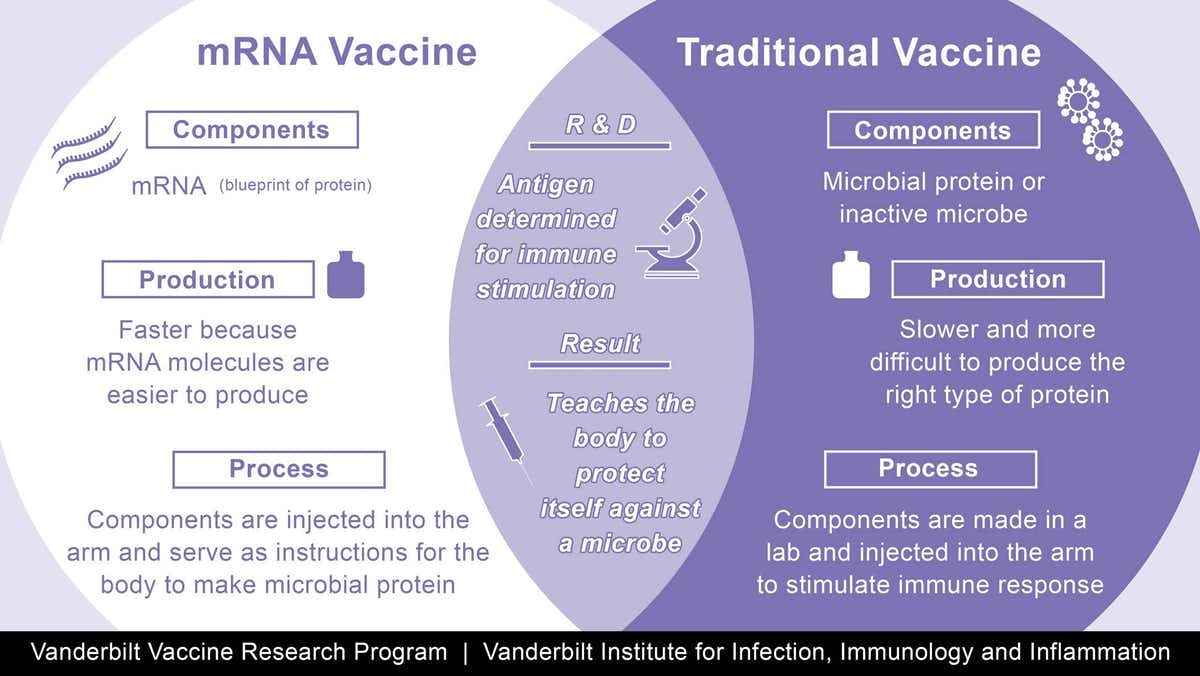
The vaccines are made using a synthetic messenger ribonucleic acid, or mRNA, that contains information about the coronavirus’s signature spike protein. When injected into the body, it provides the immune system a preview of what the threat looks like and allows it to prepare the antibodies needed to neutralize the virus if, or when, a person gets infected. This is different from traditional vaccines, which train the immune system by injecting weakened viruses into the body that cannot reproduce (or replicate) themselves very effectively.
The groundbreaking approach has several advantages. Since it does not use a live virus, there is little danger of the inoculated person being infected with the coronavirus. mRNA vaccines are also easier and faster to mass-produce than traditional options.
However, questions about how long the vaccines will provide protection and their performance across various age groups and ethnicities are yet to be answered. Also, at an expected price of between $15 to $25 per dose, the immunizations will be expensive to deploy, especially since each person needs two shots to obtain full immunity.
Additionally, Pfizer's BNT162b2 must be stored at a temperature of minus 94 degrees Fahrenheit (-70 degrees Celsius), and, once thawed, lasts just five days in regular refrigerators. This means that the countries wishing to use the vaccination will first have to build cold storage facilities. Moderna's mRNA-1273 will be slightly easier to deploy since it can be stored in a regular medical freezer for up to six months. It also remains stable in a standard refrigerator for up to a month after thawing.
Pfizer and Moderna are not the only ones scrambling to find a way to stop the spread of this deadly virus that has brought the world to a halt. Britain's AstraZeneca is also developing a vaccine that looks extremely promising. The company maintains that the vaccine will be stable for up to six months in regular refrigerators and, more importantly, will cost between just $3 to $5 a dose.
With the world's greatest minds working together, there is little doubt that the coronavirus will be eradicated. However, given the extent of its reach, it will take some time. Until then, we must all continue to take the precautions recommended by medical experts.
Stay safe and healthy
We are all in this together!
Resources: Vox.com, Observer.com, NPR.org, thehour.com, NBCnews.com
